- 1Department of Pharmacy, Xuzhou Oriental Hospital Affiliated to Xuzhou Medical University, Xuzhou, Jiangsu, China
- 2Jiangsu Key Laboratory of New Drug Research and Clinical Pharmacy & School of Pharmacy, Xuzhou Medical University, Xuzhou, Jiangsu, China
- 3Department of Pharmacy, The Affiliated Huaian NO.1 People’s Hospital of Nanjing Medical University, Huaian, Jiangsu, China
- 4Department of Pharmacy, Suzhou Research Center of Medical School, Suzhou Hospital, Affiliated Hospital of Medical School, Nanjing University, Suzhou, Jiangsu, China
- 5Department of Infection Diseases, Suzhou Research Center of Medical School, Suzhou Hospital, Affiliated Hospital of Medical School, Nanjing University, Suzhou, Jiangsu, China
- 6Department of Cardiology, Xuzhou Municipal Hospital Affiliated to Xuzhou Medical University, Xuzhou, Jiangsu, China
Objective: Olanzapine is already used to treat patients with major depressive disorder; however, whether complex drug–drug interaction (DDI) has an effect on the pharmacokinetics of people using olanzapine and its initial dosage remains unknown. The present study aims to explore the effect of DDI on olanzapine.
Methods: In total, 72 patients with major depressive disorder were included for analysis. Potential physiological and biochemical indices and other drug combination information were collected to explore the effect of clinical olanzapine concentrations by building a nonlinear mixed effect (NONMEM) model and to further simulate the optimal olanzapine initial dosage by use of the Monte Carlo method in patients with major depressive disorder.
Results: Weight and combined use of paroxetine significantly affected olanzapine clearance. With the same weight, the clearance rates of olanzapine were 0.711:1 in patients with major depressive disorder with or without paroxetine. For the initial dosages, without paroxetine, the olanzapine administration dosages, 0.5 and 0.4 mg/kg/day were recommended for patients with major depressive disorder in the groups weighing 40 to 56 kg and 56 to 100 kg, respectively. With paroxetine, olanzapine administration dosages of 0.3 and 0.2 mg/kg/day were recommended for patients with major depressive disorder in the groups weighing 40 to 85 kg and 85 to 100 kg, respectively.
Conclusions: This has been the first case to establish olanzapine population pharmacokinetics in patients with major depressive disorder. In addition, the present study innovatively clarified that paroxetine affected olanzapine population pharmacokinetics and initial dosage in patients with major depressive disorder.
1 Introduction
Major depressive disorder is characterized by widespread and lasting depression and loss of interest manifested as low mood, pessimism, and depression accompanied by memory loss, fatigue, gastrointestinal discomfort, cognitive impairment, and other symptoms, resulting in a decline in physical and social function (1, 2). The most dangerous clinical symptom of major depressive disorder is suicide; the rate of suicide is 20 times that of people without major depressive disorder (3). Previous studies have shown that major depressive disorder has become the second most common disease after cardiovascular disease (4). It significantly reduces the quality of life and not only increases the mental burden of individuals but also the incidence and mortality of other diseases, such as cardiovascular disease and diabetes, leading to an increase in medical costs and further aggravating the economic burden of society (5, 6). The recent consensus statement on treatment-resistant depression by Maina et al. contextualizes the challenges of treatment-resistant depression and the need for alternative strategies (7).
At this stage, drug therapy is the first choice for the treatment of major depressive disorder, and the commonly used drugs for major depressive disorder mainly include selective serotonin and noradrenaline reuptake inhibitors, monoamine oxidase inhibitors, tricyclic antidepressants, and multimodal drugs. Olanzapine is an atypical antipsychotic medication widely used in the treatment of various psychiatric disorders. Its primary indications include schizophrenia, bipolar disorder, anxiety, and depression. The sedative effect of olanzapine is significantly stronger than that of aripiprazole; the sedative effect of two aripiprazole tablets was equivalent to one olanzapine tablet at a clinical equivalent dosage. Studies have shown that olanzapine can provide more benefits in the multi-drug combination of major depressive disorder (8–13). Olanzapine could be used as an alternative to lithium as an option for patients with major depressive disorder who do not respond to paroxetine treatment (12). In addition, usage of an olanzapine-fluoxetine combination in major depressive disorder has been reported (14, 15). However, olanzapine is greatly affected by drug–drug interactions (DDI) in clinical practice, and variation in dosage or drug concentration levels easily affects efficacy or results in adverse reactions (16–19). Several drugs exhibit interactions with olanzapine, such as fluvoxamine, fluoroquinolones, antiretroviral drugs, propafenone and flecainide, fluoxetine, and duloxetine (20–25). How to identify the factors affecting olanzapine and formulate an appropriate olanzapine administration regime for patients with major depressive disorder has become an urgent problems in clinical practice.
Population pharmacokinetics employs a nonlinear mixed-effects model to quantitatively characterize the absorption, distribution, metabolism, and excretion processes of drugs within populations, analyze inter-individual variability in pharmacokinetic parameters, and investigate the impact of covariates. The present study aims to collect potential physiological and biochemical indices and drug combination information to explore the effect on clinical olanzapine concentrations and to further simulate the optimal olanzapine initial dosage by using population pharmacokinetics and the Monte Carlo method, innovatively clarifying how DDI affects olanzapine population pharmacokinetics and initial dosage in patients with major depressive disorder.
2 Methods
2.1 Data collection
We collected data on patients with major depressive disorder who were hospitalized and treated with olanzapine at Xuzhou Oriental Hospital affiliated to Xuzhou Medical University, between December 2020 and August 2023, retrospectively. The inclusion criteria were as follows: (i) patients with major depressive disorder, (ii) olanzapine treatment, (iii) carrying out therapeutic drug monitoring (TDM) for olanzapine regularly, and (iv) a detailed treatment plan. Potential physiological and biochemical indices (which were obtained from the patient’s medical record system, and the detection of these indicators was carried out by the hospital laboratory according to the clinical diagnosis and treatment path, conventional), drug combination information, and olanzapine concentrations were collected. The above research was approved by the Research Ethics Committee of the Xuzhou Oriental Hospital affiliated to Xuzhou Medical University (No.20220725011), where the requirement for written informed consent could be waived since the data were collected without patient identifiers.
2.2 Modeling
In the modeling process of this study, apparent oral clearance (CL/F), volume of distribution (V/F), and absorption rate constants [Ka, fixed at 0.861/h (26)] were taken into consideration. In addition, the olanzapine population pharmacokinetic model in patients with major depressive disorder was built up using non-linear mixed effect modeling (NONMEM, version 7, ICON Development Solutions, Ellicott City, MD, USA) software.
Equation 1 shows inter-individual variability:
Ai is the individual parameter value. TV(A) is the typical individual parameter value. ηi is the symmetrical distribution, which was a random term with zero mean and variance omega^2 (ω2).
Equation 2 shows the random residual variability:
Bi is the observed concentration. Ci is the individual predicted concentration. ϵn is the symmetrical distribution, which was a random term with zero mean and variance sigma^2 (σ2).
Equation 3 shows the relationship of pharmacokinetic parameters with weight:
Di is the i-th individual parameter. Ei is the i-th individual weight. Estd is the standard weight of 70 kg. Dstd is the typical individual parameter whose weight was Estd. F is the allometric coefficient: 0.75 for the CL/F and 1 for the V/F (27).
Equations 4, 5 show the pharmacokinetic parameters between continuous covariates and categorical covariates, respectively:
Gi is the individual parameter value. TV(G) is the typical individual parameter value. θ is the parameter to be estimated. Covi is the covariate of the i-th individual. Covm is the population median for the covariate.
The covariate model was constructed in a stepwise way. Potential covariates included physiological and biochemical indices and drug combinations. The objective function value (OFV) variation was covariate inclusion criteria, among which OFV decrease>3.84 (P<0.05) was defined as the inclusion standard, and OFV increase>6.63 (P<0.01) was defined as the exclusion standard.
2.3 Model validation
Observations vs. population predictions, observations vs. individual predictions, absolute value of weighted residuals of individual (│iWRES│) vs. individual predictions, weighted residuals vs. time, density vs. weighted residuals, quantiles of weighted residuals vs. quantiles of normal, and visual predictive check (VPC) of the model and individual plot were used to evaluate the final model. Besides, the bootstrap method was used to compare with the final model parameters.
2.4 Simulation
Initial dosage optimization of olanzapine in patients with major depressive disorder was carried out using Monte Carlo simulation, where the olanzapine therapeutic window was 20 to 80 ng/ml (28). The present study found that weight and the combined use of paroxetine significantly affected olanzapine clearance. Therefore, according to whether paroxetine was used in combination or not, and as a once-daily or a twice-daily olanzapine (split evenly into two dosages a day) dose, we simulated four different cases; every case had 1000 virtual patients with major depressive disorder, 10 dosages (0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7, 0.8, 0.9, and 1.0 mg/kg/day) for seven weight groups (40, 50, 60, 70, 80, 90, and 100 kg). In the present study, the probability of achieving the target concentration was selected as the evaluation criterion.
3 Results
3.1 Patient information
Demographic data of patients with major depressive disorder are shown in Table 1: 72 patients with major depressive disorder (the concentration samples per patient were 1-3), 17 male and 55 female, whose ages ranged from 16.00 to 87.90 years old and weights were from 40.00 to 92.00 kg. Drug combination in patients with major depressive disorder are shown in Table 2, including atorvastatin calcium tablets, alprazolam tablets, amlodipine besylate tablets, benzoxol hydrochloride tablets, buspirone hydrochloride tablets, clonazepam tablets, dexzopiclone, duloxetine hydrochloride enteric-coated capsules, enteric-coated aspirin, escitalopram oxalate tablets, irbesartan hydrochlorothiazide tablets, levodopa and benserazide tablets, lorazepam tablets, metoprolol succinate tablets, mirtazapine tablets, omeprazole enteric-coated capsules, oxazepam, paroxetine hydrochloride tablets, propranolol hydrochloride tablets, sertraline hydrochloride tablets, trazodone hydrochloride tablets, valsartan capsules, venlafaxine hydrochloride tablets, and zopiclone tablets.
3.2 Modeling
The final model of olanzapine in patients with major depressive disorder was shown in Equations 6, 7:
CL/F represents apparent oral clearance. V/F represents apparent volume of distribution. PAR represents paroxetine; when patients took paroxetine, PAR was 1, otherwise PAR was 0.
3.3 Evaluation
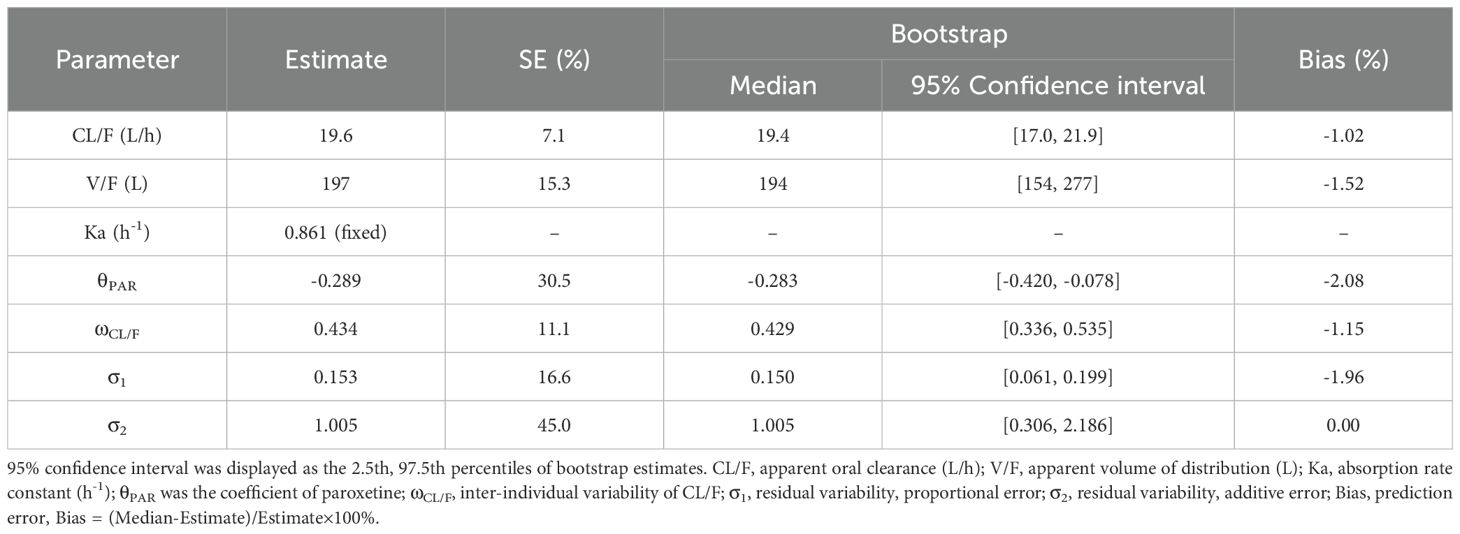
Figures 1A-G shows observations vs. population predictions, observations vs. individual predictions,│iWRES│vs. individual predictions, weighted residuals vs. time, density vs. weighted residuals, quantiles of weighted residuals vs. quantiles of normal, and VPC of the model. These results suggested that the final model predicted well. Figure 1H shows that with the same weight, the clearance rates of olanzapine were 0.711:1 in patients with major depressive disorder with or without paroxetine. Figure 2 shows the individual plot, and from a clinical standpoint, our final model could predict the olanzapine concentrations of patients well at the individual level. Table 3 shows the parameter estimate of the final model and bootstrap validation, indicating the final model was accurate and reliable.

Figure 1. Model evaluation. (A) Observations vs. population predictions. (B) Observations vs. individual predictions. (C) absolute value of weighted residuals of individual (│iWRES│) vs. individual predictions. (D) Weighted residuals vs. time. (E) Density vs. weighted residuals. (F) Quantiles of weighted residuals vs. quantiles of normal. (G) Visual predictive check (VPC) of the model. (H) Olanzapine clearance. a: without paroxetine, b: with paroxetine.

Figure 2. Individual plot. ID, patient ID number; DV, measured concentration value; IPRED, individual predictive value; PRED, population predictive value.
3.4 Simulation
The simulated olanzapine concentrations of once-daily olanzapine administration dosages without paroxetine, twice-daily olanzapine administration dosages without paroxetine, once-daily olanzapine administration dosages with paroxetine, and twice-daily olanzapine administration dosages with paroxetine are shown in Figures 3A–D, respectively. Each colorful box diagram represents the predicted olanzapine trough levels of the corresponding dosage. The two red dashed lines represent the olanzapine therapeutic window (20–80 ng/ml), and the parts within the upper and lower red dashed lines represent concentrations reaching the therapeutic window. The probabilities of achieving the target concentration from once-daily olanzapine administration dosages without paroxetine, twice-daily olanzapine administration dosages without paroxetine, once-daily olanzapine administration dosages with paroxetine, and twice-daily olanzapine administration dosages with paroxetine are shown in Figures 4A–D, respectively. Based on simulation results, Table 4 shows the optimal olanzapine initial dosages in patients with major depressive disorder. Without paroxetine, for once-daily olanzapine administration dosages, the probability for achieving the target concentrations from all dosages (0.1–1.0 mg/kg/day) was less than 55.0%. For twice-daily olanzapine administration dosages, 0.5 and 0.4 mg/kg/day were recommended for patients with major depressive disorder weighing 40 to 56 kg and 56 to 100 kg, respectively. Meanwhile, the probabilities of achieving target concentrations at these dosages were 68.5 to 68.9% and 68.5 to 72.6%, respectively. With paroxetine, for once-daily olanzapine administration dosages, 0.5 and 0.4 mg/kg/day were recommended for patients with major depressive disorder weighing 40 to 60 kg and 60 to 100 kg, respectively. Meanwhile, the probabilities of achieving target concentrations at these dosages were 57.0 to 59.0% and 58.8 to 62.1%, respectively. For twice-daily olanzapine administration dosages, 0.3 and 0.2 mg/kg/day were recommended for patients with major depressive disorder weighing 40 to 85 kg and 85 to 100 kg, respectively. Meanwhile, the probabilities of achieving target concentrations at these dosages were 74.4 to 76.7% and 75.1 to 76.9%, respectively.

Figure 3. Simulated olanzapine concentrations. (A) Once-daily olanzapine administration dosages without paroxetine. (B) Twice-daily olanzapine administration dosages without paroxetine. (C) Once-daily olanzapine administration dosages with paroxetine. (D) Twice-daily olanzapine administration dosages with paroxetine. a: patients with major depressive disorder (40 kg), b: patients with major depressive disorder (50 kg), c: patients with major depressive disorder (60 kg), d: patients with major depressive disorder (70 kg), e: patients with major depressive disorder (80 kg), f: patients with major depressive disorder (90 kg), g: and patients with major depressive disorder (100 kg). The lower and upper red dashed lines were 20 and 80 ng/ml, respectively.

Figure 4. Probabilities for achieving a therapeutic window. (A) Once-daily olanzapine administration dosages without paroxetine. (B) Twice-daily olanzapine administration dosages without paroxetine. (C) Once-daily olanzapine administration dosages with paroxetine. (D) Twice-daily olanzapine administration dosages with paroxetine.

Table 4. Initial dosage recommendation of olanzapine in patients with major depressive disorder with or without paroxetine.
4 Discussion
The involvement of olanzapine in the treatment of patients with major depressive disorder has been widely reported (8–13), and researchers have shown that patients with major depressive disorder can receive more benefits from treatment with olanzapine. However, DDI may greatly affect the metabolism and formulation of the dosage regimen of olanzapine. In clinical practice, how to explore the influencing factors of olanzapine, quantify the degree of influence, and then formulate an optimal olanzapine dosage is urgent. TDM is guided by the basic theory of pharmacokinetics and pharmacodynamics, with the help of advanced analysis technology and electronic computer means, and the use of pharmacokinetic principles and formulas to individualize the drug delivery program (29–33). The blood concentration reported by TDM can provide a clinical basis for the next adjustment of the olanzapine administration schedule in patients. Nevertheless, due to the lack of blood concentration information, TDM alone cannot provide a reference for the initial dosage of olanzapine in patients with major depressive disorder.
Luckily, the combination of population pharmacokinetics and Monte Carlo simulation can make more full use of information from clinical TDM and provide references for initial drug administration recommendations through machine learning techniques. There has been considerable practice in this area, particularly focusing on DDIs. For example, Cai et al. found that voriconazole concomitant therapy affected tacrolimus in lung transplant recipients; meanwhile, the dosing regimen of tacrolimus was recommended based on whether voriconazole was combined (34). Chen et al. reported effects of posaconazole on tacrolimus population pharmacokinetics and initial dose in children with Crohn’s disease undergoing hematopoietic stem cell transplantation (35). Wang et al. reported effects of cimetidine on ciclosporin population pharmacokinetics and initial dose optimization in aplastic anemia patients (36). Chen et al. reported effects of voriconazole on population pharmacokinetics and optimization of the initial dose of tacrolimus in children with chronic granulomatous disease undergoing hematopoietic stem cell transplantation (37). Thus, the present study aims to explore the effect of DDI on olanzapine using population pharmacokinetics and Monte Carlo simulation.
In the present study, 72 patients with major depressive disorder were included, and potential physiological and biochemical indices and drug combination information were collected to explore the effect of olanzapine on clinical concentrations. Finally, weight and the combined use of paroxetine significantly affected olanzapine clearance. Paroxetine is a potent inhibitor of the CYP2D6 enzyme, and olanzapine is metabolized by the CYP2D6 enzyme, and then paroxetine inhibits the metabolism of olanzapine by inhibiting the CYP2D6 enzyme (38–44). With the same weight, the clearance rates of olanzapine were 0.711:1 in patients with major depressive disorder with or without paroxetine. Further, we simulated once-daily or twice-daily olanzapine administration dosages, among which twice daily was optimal. For the initial dosage of twice daily, without paroxetine, the olanzapine administration dosages 0.5 and 0.4 mg/kg/day were recommended for patients with major depressive disorder weighing 40 to 56 kg and 56 to 100 kg, respectively. With paroxetine, olanzapine administration dosages of 0.3 and 0.2 mg/kg/day were recommended for patients with major depressive disorder weighing 40 to 85 kg and 85 to 100 kg, respectively.
In addition, in a previous study, we used a similar research method to explore olanzapine population pharmacokinetics and initial dosage optimization in patients with schizophrenia, where 65 patients with schizophrenia were enrolled for analysis (45). In that study, we found that the combined use of aripiprazole significantly affected olanzapine clearance. Without aripiprazole, for twice-daily olanzapine administration dosages, 0.6 and 0.5 mg/kg/day were recommended for patients with schizophrenia weighing 40 to 60 kg and 60 to 100 kg, respectively. With aripiprazole, for twice-daily olanzapine administration dosages, 0.4 mg/kg/day was recommended for patients with schizophrenia weighing 40 to 100 kg (45). In summary, we have completed the precision administration and dosage recommendation of olanzapine in two independent populations: patients with schizophrenia and patients with major depressive disorder. In the future, we will further explore the precise administration and dosage recommendation of olanzapine in other populations.
Certainly, this study has limitations, such as the retrospective data, relatively small sample size, and insufficient in-depth exploration of patients’ dietary habits and comorbid diseases. Future research should conduct a prospective study with larger sample sizes and more comprehensive investigations into additional potential influencing factors.
5 Conclusion
This is the first study to establish olanzapine population pharmacokinetics in patients with major depressive disorder. In addition, the present study innovatively clarified that paroxetine affected olanzapine population pharmacokinetics and the initial dosage for patients with major depressive disorder.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Research Ethics Committee of the Xuzhou Oriental Hospital Affiliated to Xuzhou Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because The data were collected retrospectively without patient identifiers.
Author contributions
CZ: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft. LC: Data curation, Formal Analysis, Investigation, Methodology, Software, Validation, Writing – original draft. Y-YD: Data curation, Formal Analysis, Methodology, Project administration, Supervision, Validation, Writing – original draft. S-MH: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing. Y-LT: Conceptualization, Writing – review & editing. YG: Conceptualization, Writing – review & editing. D-DW: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by The Xuzhou Special Fund for Promoting Scientific and Technological Innovation (No. KC23254, No. KC23217), The Medical Research Project of Jiangsu Provincial Health Commission (No. Z2023010), Jiangsu Province Education Science Planning Project (No. C/2022/01/36), Xuzhou Medical University Labor Education Special Project (No. X1d202209), Jiangsu Province Higher Education Informatization Research Topic (2023JSETKT136), and Xuzhou Medical University Research Topic of Higher Education Teaching Reform (Xjyzrd202304).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2025.1538996/full#supplementary-material
Supplementary Table 1 | Effect of drug interaction and gender on olanzapine in patients with major depressive disorder.
References
1. D'Souza S, Thompson JM, Slykerman R, Marlow G, Wall C, Murphy R, et al. Environmental and genetic determinants of childhood depression: The roles of DAT1 and the antenatal environment. J Affect Disord. (2016) 197:151–8. doi: 10.1016/j.jad.2016.03.023
2. Qi D, Chen K. Bioinformatics analysis of potential biomarkers and pathway identification for major depressive disorder. Comput Math Methods Med. (2021) 2021:3036741. doi: 10.1155/2021/3036741
3. Li H, Chen Z, Gong Q, Jia Z. Voxel-wise meta-analysis of task-related brain activation abnormalities in major depressive disorder with suicide behavior. Brain Imaging Behav. (2020) 14:1298–308. doi: 10.1007/s11682-019-00045-3
4. Zeng Y, Navarro P, Shirali M, Howard DM, Adams MJ, Hall LS, et al. Genome-wide regional heritability mapping identifies a locus within the TOX2 gene associated with major depressive disorder. Biol Psychiatry. (2017) 82:312–21. doi: 10.1016/j.biopsych.2016.12.012
5. Weissman MM, Wickramaratne P, Pilowsky DJ, Poh E, Hernandez M, Batten LA, et al. The effects on children of depressed mothers' remission and relapse over 9 months. Psychol Med. (2014) 44:2811–24. doi: 10.1017/S003329171400021X
6. Zhang Y, Chen Y, Ma L. Depression and cardiovascular disease in elderly: Current understanding. J Clin Neurosci. (2018) 47:1–5. doi: 10.1016/j.jocn.2017.09.022
7. Maina G, Adami M, Ascione G, Bondi E, De Berardis D, Delmonte D, et al. Nationwide consensus on the clinical management of treatment-resistant depression in Italy: a Delphi panel. Ann Gen Psychiatry. (2023) 22:48. doi: 10.1186/s12991-023-00478-7
8. Barbee JG, Conrad EJ, Jamhour NJ. The effectiveness of olanzapine, risperidone, quetiapine, and ziprasidone as augmentation agents in treatment-resistant major depressive disorder. J Clin Psychiatry. (2004) 65:975–81. doi: 10.4088/jcp.v65n0714
9. Mathews J, Garcia KS, Mintun MA, Sheline YI. Antidepressant efficacy of olanzapine as monotherapy in major depressive disorder, without psychosis: a pilot study. Psychiatry Res. (2006) 146:149–55. doi: 10.1016/j.pscychresns.2005.08.003
10. Brunner E, Tohen M, Osuntokun O, Landry J, Thase ME. Efficacy and safety of olanzapine/fluoxetine combination vs fluoxetine monotherapy following successful combination therapy of treatment-resistant major depressive disorder. Neuropsychopharmacology. (2014) 39:2549–59. doi: 10.1038/npp.2014.101
11. Lin CY, Tsai GE, Wang HS, Wu YH, Chiou CC, Wu VY, et al. Effectiveness of aripiprazole, olanzapine, quetiapine, and risperidone augmentation treatment for major depressive disorder: a nationwide population-based study. J Clin Psychiatry. (2014) 75:e924–931. doi: 10.4088/JCP.13m08843
12. Yoshimura R, Hori H, Umene-Nakano W, Ikenouchi-Sugita A, Katsuki A, Atake K, et al. Comparison of lithium, aripiprazole and olanzapine as augmentation to paroxetine for inpatients with major depressive disorder. Ther Adv Psychopharmacol. (2014) 4:123–9. doi: 10.1177/2045125313514767
13. Zhong Z, Zhang Y, Han H, Huang Z, Wang J, Chen M, et al. Effects of low-dose olanzapine on duloxetine-related nausea and vomiting for the treatment of major depressive disorder. J Clin Psychopharmacol. (2014) 34:495–8. doi: 10.1097/JCP.0000000000000167
14. Rothschild AJ, Williamson DJ, Tohen MF, Schatzberg A, Andersen SW, Van Campen LE, et al. A double-blind, randomized study of olanzapine and olanzapine/fluoxetine combination for major depression with psychotic features. J Clin Psychopharmacol. (2004) 24:365–73. doi: 10.1097/01.jcp.0000130557.08996.7a
15. Bobo WV, Shelton RC. Fluoxetine and olanzapine combination therapy in treatment-resistant major depression: review of efficacy and safety data. Expert Opin Pharmacother. (2009) 10:2145–59. doi: 10.1517/14656560903130609
16. Deng M, Yang Z, Ni Y, Zhu L, Xu J, Zheng L, et al. Effects of varenicline on the serum levels of olanzapine in male patients with Schizophrenia: a randomized controlled trial. Front Psychiatry. (2023) 14:1142419. doi: 10.3389/fpsyt.2023.1142419
17. Fekete F, Menus A, Toth K, Kiss AF, Minus A, Sirok D, et al. CYP1A2 expression rather than genotype is associated with olanzapine concentration in psychiatric patients. Sci Rep. (2023) 13:18507. doi: 10.1038/s41598-023-45752-6
18. Horvat M, Kadija M, Scavnicar A, Zivkovic M, Sagud M, Lovric M. Association of smoking cigarettes, age, and sex with serum concentrations of olanzapine in patients with schizophrenia. Biochem Med (Zagreb). (2023) 33:30702. doi: 10.11613/BM.2023.030702
19. Mao JH, Han L, Liu XQ, Jiao Z. Significant predictors for olanzapine pharmacokinetics: a systematic review of population pharmacokinetic studies. Expert Rev Clin Pharmacol. (2023) 16:575–88. doi: 10.1080/17512433.2023.2219055
20. Markowitz JS, DeVane CL. Suspected ciprofloxacin inhibition of olanzapine resulting in increased plasma concentration. J Clin Psychopharmacol. (1999) 19:289–91. doi: 10.1097/00004714-199906000-00023
21. Gossen D, de Suray JM, Vandenhende F, Onkelinx C, Gangji D. Influence of fluoxetine on olanzapine pharmacokinetics. AAPS PharmSci. (2002) 4:E11. doi: 10.1208/ps040211
22. Bergemann N, Frick A, Parzer P, Kopitz J. Olanzapine plasma concentration, average daily dose, and interaction with co-medication in schizophrenic patients. Pharmacopsychiatry. (2004) 37:63–8. doi: 10.1055/s-2004-815527
23. Englisch S, Fritzinger M, Zink M. Urinary retention during combined treatment of postpsychotic depression with duloxetine and olanzapine. Clin Neuropharmacol. (2008) 31:307–9. doi: 10.1097/WNF.0b013e318157e462
24. Jacobs BS, Colbers AP, Velthoven-Graafland K, Schouwenberg BJ, Burger DM. Effect of fosamprenavir/ritonavir on the pharmacokinetics of single-dose olanzapine in healthy volunteers. Int J Antimicrob Agents. (2014) 44:173–7. doi: 10.1016/j.ijantimicag.2014.03.014
25. Siwek M, Woron J, Gorostowicz A, Wordliczek J. Adverse effects of interactions between antipsychotics and medications used in the treatment of cardiovascular disorders. Pharmacol Rep. (2020) 72:350–9. doi: 10.1007/s43440-020-00058-6
26. Sun L, Mills R, Sadler BM, Rege B. Population pharmacokinetics of olanzapine and samidorphan when administered in combination in healthy subjects and patients with schizophrenia. J Clin Pharmacol. (2021) 61:1430–41. doi: 10.1002/jcph.1911
27. Anderson BJ, Holford NH. Mechanism-based concepts of size and maturity in pharmacokinetics. Annu Rev Pharmacol Toxicol. (2008) 48:303–32. doi: 10.1146/annurev.pharmtox.48.113006.094708
28. Ding J, Zhang Y, Zhang Y, Yang L, Zhang S, Cui X, et al. Effects of age, sex, and comedication on the plasma concentrations of olanzapine in chinese patients with schizophrenia based on therapeutic drug monitoring data. J Clin Psychopharmacol. (2022) 42:552–9. doi: 10.1097/JCP.0000000000001618
29. Engels FK, Loos WJ, van der Bol JM, de Bruijn P, Mathijssen RH, Verweij J, et al. Therapeutic drug monitoring for the individualization of docetaxel dosing: a randomized pharmacokinetic study. Clin Cancer Res. (2011) 17:353–62. doi: 10.1158/1078-0432.CCR-10-1636
30. Abrantes JA, Jonsson S, Karlsson MO, Nielsen EI. Handling interoccasion variability in model-based dose individualization using therapeutic drug monitoring data. Br J Clin Pharmacol. (2019) 85:1326–36. doi: 10.1111/bcp.13901
31. Maillard M, Le Louedec F, Thomas F, Chatelut E. Diversity of dose-individualization and therapeutic drug monitoring practices of platinum compounds: a review. Expert Opin Drug Metab Toxicol. (2020) 16:907–25. doi: 10.1080/17425255.2020.1789590
32. Baymeeva NV, Miroshnichenko II, Platova AI, Tikhonov DV, Kaleda VG. Therapeutic drug monitoring of aripiprazole as part of the individualization of the pharmacotherapy of schizophrenia. Zh Nevrol Psikhiatr Im S S Korsakova. (2022) 122:98–103. doi: 10.17116/jnevro202212201198
33. Lin B, Hu Y, Xu P, Xu T, Chen C, He L, et al. Expert consensus statement on therapeutic drug monitoring and individualization of linezolid. Front Public Health. (2022) 10:967311. doi: 10.3389/fpubh.2022.967311
34. Cai X, Song H, Jiao Z, Yang H, Zhu M, Wang C, et al. Population pharmacokinetics and dosing regimen optimization of tacrolimus in Chinese lung transplant recipients. Eur J Pharm Sci. (2020) 152:105448. doi: 10.1016/j.ejps.2020.105448
35. Chen X, Wang D, Zheng F, Zhu L, Huang Y, Zhu Y, et al. Effects of posaconazole on tacrolimus population pharmacokinetics and initial dose in children with crohn's disease undergoing hematopoietic stem cell transplantation. Front Pharmacol. (2022) 13:758524. doi: 10.3389/fphar.2022.758524
36. Wang DD, He SM, Yang Y, Mao YZ, Yin D, Zheng ZQ, et al. Effects of cimetidine on ciclosporin population pharmacokinetics and initial dose optimization in aplastic anemia patients. Eur J Pharm Sci. (2022) 174:106183. doi: 10.1016/j.ejps.2022.106183
37. Chen X, Wang D, Lan J, Wang G, Zhu L, Xu X, et al. Effects of voriconazole on population pharmacokinetics and optimization of the initial dose of tacrolimus in children with chronic granulomatous disease undergoing hematopoietic stem cell transplantation. Ann Transl Med. (2021) 9:1477. doi: 10.21037/atm-21-4124
38. Zhou HY, Gu EM, Chen QL, Zhan YY, Wang SH, Liang BQ, et al. Effects of 22 CYP2D6 genetic variations newly identified in chinese population on olanzapine metabolism in vitro. Pharmacology. (2016) 98:124–33. doi: 10.1159/000446807
39. Jung EH, Lee YJ, Kim DH, Kang P, Lim CW, Cho CK, et al. Effects of paroxetine on the pharmacokinetics of atomoxetine and its metabolites in different CYP2D6 genotypes. Arch Pharm Res. (2020) 43:1356–63. doi: 10.1007/s12272-020-01300-8
40. Soria-Chacartegui P, Villapalos-Garcia G, Zubiaur P, Abad-Santos F, Koller D. Genetic polymorphisms associated with the pharmacokinetics, pharmacodynamics and adverse effects of olanzapine, aripiprazole and risperidone. Front Pharmacol. (2021) 12:711940. doi: 10.3389/fphar.2021.711940
41. Wang Z, Kosheleff AR, Adeojo LW, Odebo O, Adewole T, Qin P, et al. Impact of paroxetine, a strong CYP2D6 inhibitor, on SPN-812 (Viloxazine extended-release) pharmacokinetics in healthy adults. Clin Pharmacol Drug Dev. (2021) 10:1365–74. doi: 10.1002/cpdd.948
42. Rojas-Macetas A, Medalla-Garro G, Saravia M, Losno R, Valderrama-Wong M, Pariona R, et al. Potential polymorphic CYP1A2 and CYP2D6-mediated pharmacokinetic interactions between risperidone or olanzapine and selected drugs intended to treat COVID-19. Drug Metab Bioanal Lett. (2022) 16(1):6–13. doi: 10.2174/1872312815666221125112724
43. Rudesheim S, Selzer D, Murdter T, Igel S, Kerb R, Schwab M, et al. Physiologically based pharmacokinetic modeling to describe the CYP2D6 activity score-dependent metabolism of paroxetine, atomoxetine and risperidone. Pharmaceutics. (2022) 14(8):1734. doi: 10.3390/pharmaceutics14081734
44. Djerada Z, Brousse G, Niel P, Llorca PM, Eschalier A, Bentue-Ferrer D, et al. Therapeutic drug monitoring of olanzapine. Therapie. (2023) 78:S75–80. doi: 10.2515/therapie/2015040
Keywords: drug-drug interaction, paroxetine, olanzapine, population pharmacokinetics, initial dosage, major depressive disorder
Citation: Zhang C, Chen L, Duan Y-Y, He S-M, Tian Y-L, Gao Y and Wang D-D (2025) Drug-drug interaction of paroxetine on olanzapine and initial dosage optimization in patients with major depressive disorder based on population pharmacokinetics. Front. Psychiatry 16:1538996. doi: 10.3389/fpsyt.2025.1538996
Received: 03 December 2024; Accepted: 28 April 2025;
Published: 13 May 2025.
Edited by:
YiPing Liu, Central South University, ChinaReviewed by:
Andy R. Eugene, Osawatomie State Hospital, United StatesMarcin Siwek, Jagiellonian University, Poland
Magdalena Sowa-Kućma, University of Rzeszow, Poland
Vassilis Martiadis, Asl Napoli 1 Centro, Italy
Copyright © 2025 Zhang, Chen, Duan, He, Tian, Gao and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Su-Mei He, aGVoZTgyMDRAMTYzLmNvbQ==; Ya-Li Tian, dHlsNDkyMTlAMTYzLmNvbQ==; Ying Gao, eHp5eWdhb3lpbmdAMTYzLmNvbQ==; Dong-Dong Wang, MTM4NTIwMjk1OTFAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Cun Zhang
Cun Zhang Liang Chen2†
Liang Chen2† Su-Mei He
Su-Mei He Dong-Dong Wang
Dong-Dong Wang